RESEARCH
CARROLL PROJECT STEVENS/JOHNSON PROJECT SABATINI PROJECTGRANGER/JOHNSON PROJECT
Psychiatric Risk Factors and Neurodevelopment


Principal Investigator for the Conte Center
Project Scientists
The human brain continues to develop and mature throughout adolescence, with the association areas of the cerebral cortex not maturing until two decades after birth.
Protracted postnatal development extends the brain’s period of plasticity and enables humans to master advanced cognitive skills such as language, abstraction, and complex social interactions. However, prolonged plasticity also appears to increase our vulnerability to neurodevelopmental disorders that could disrupt these same cognitive domains. The final epoch of brain development, late adolescence, coincides with a period of increased incidence of major mental illnesses including schizophrenia and bipolar disorder. This suggests that genetic risk factors preferentially impact processes at work in this late postnatal developmental period such as synaptic pruning. Furthermore, childhood adversity – e.g., trauma, abuse, or parental neglect – is consistently associated with increased schizophrenia risk, highlighting the vulnerability of postnatal brain development to environmental challenges.
Genetic Risk Factor for Schizophrenia
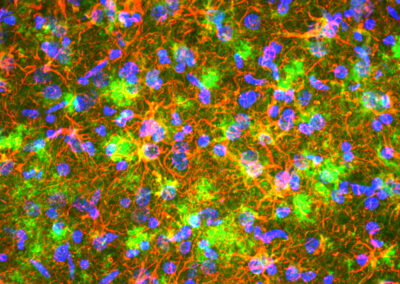
In health, Complement C4 (green) is restricted to blood vessels, but during neurodegeneration, astrocytes (red) become reactive and secrete C4 into the brain tissue.
Viral Expression of Human Complement
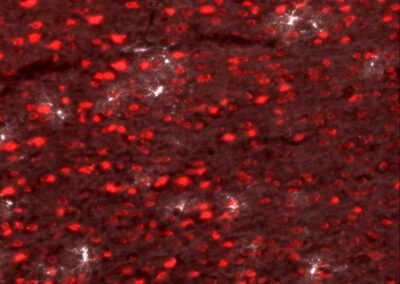
By injecting mice with virus, the expression of human complement 4 (hC4; white) can be controlled in the neurons (red) of mice.
Given the growing evidence that neuroimmune mechanisms regulate synaptic pruning and circuit refinement in mice, we are particularly interested in better understanding when and how genetic and environmental risk factors interact with neuroimmune signals and neuron-glia communications to impact brain development and plasticity. We will investigate adolescent neurodevelopmental vulnerability to genetic and environmental risk factors using a new conditional hC4A expression mouse line, a social isolation-based chronic stressor, labeling of synaptic subpopulations in vivo, and a novel method for assaying microglial synaptic engulfment by flow cytometry. We will pursue mechanistic insights into the cellular functions of C4A and another complement-regulatory schizophrenia risk gene product using neural culture systems derived from human stem cells. Finally, we will lay the foundations for translating the cellular, physiological, and behavioral work to the common marmoset.