RESEARCH
CARROLL PROJECT STEVENS/JOHNSON PROJECT SABATINI PROJECTGRANGER/JOHNSON PROJECT
GRANGER/JOHNSON PROJECT
Psychiatric Risk Factors and Cell Maturation

Investigator for the Conte Center

Principal Investigator for the Conte Center
Project Scientists
Astrocytes play critical roles in regulating synapses and synaptic connectivity, supporting the function of synapses with physiological activities ranging from glutamate uptake to cholesterol synthesis.
Astrocytes also provide secreted and contact-dependent molecular cues that instruct the formation and elimination of synapses. Recent genetic studies have identified rare but highly penetrant gene mutations that offer a strong mechanistic foothold into the pathogenesis of psychiatric disorders. These protein-altering and loss-of-function mutations are associated, at genome-wide significance, with dramatically increased risk for schizophrenia. While several of these genes have clear roles in neuronal and synaptic function, many of them have no obvious specialized neuronal function, and the effect these genes have on the development of synapses and circuits is unknown. In addition, several of these genes are highly expressed in astrocytes and microglia, indicating the potential involvement of neuro-immune pathways in the disease.
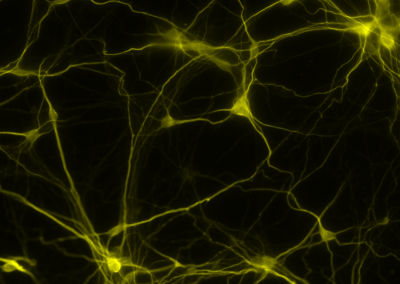
Staining Neurons in Culture
By staining for the synaptic marker MAP2, human neurons (yellow) in co-culture with mouse astrocytes can be visualized.
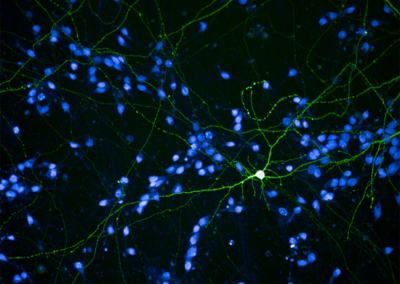
Morphological Analysis of Neurons
Human neurons (green) in culture were labeled by a low-efficacy transfection using a plasmid encoding a GFP marker to analyze their morphology, while the nuclei (blue) of all the cells were counterstained with Hoescht.
We leverage the experimental techniques, assays, and model systems being used across the Center to investigate new schizophrenia risk genes, and their relationships to neuron-astrocyte signaling, synaptic and circuit development, and neuroimmune mechanisms of psychiatric risk. We will test the involvement of risk genes in the maturation of human stem cell derived neurons and astrocytes and explore their influence on each other in collaboration with and using methods developed by the Stevens lab. We will then characterize changes to cellular signaling pathways using spatial transcriptomics (MERFISH), leveraging the gene set pathway established by the Carroll lab, as well as the developmental time- course of synapse maturation in mutant variants of risk genes mice. Finally, we will also study the neuro-immune and behavioral consequences of exposure to stress in mice carrying the risk gene mutations, to determine whether increased susceptibility to environmental challenges underlies the risk for psychiatric disease conferred by these genes. Future studies will then expand these experiments to other schizophrenia risk genes that may be involved in neuroimmune interactions.